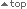Fig. 1: Flow diagram of the PCR survey
- European Treatment and Outcome Study
- Registry
- Molecular Monitoring
- Path to cure
- Spread of Excellence
Molecular Monitoring Survey - EUREKA
EUropean survey on the assessment of deep molecular REsponse in chronic phase CML patients after at least two years of therapy with tyrosine KinAse inhibitors - major component of the Path to Cure Pillar of EUTOS III
Improving the monitoring of deeper and sustained molecular responses is critical for the optimal management of BCR-ABL+ CML patients. Standardized molecular monitoring and the precise detection of deep molecular response is a prerequisite for any attempt of treatment discontinuation in CML patients (MR4.5). In this registry, BCR-ABL transcript levels after at least two years of TKI therapy will be evaluated for the occurrence of deeper molecular response rates and its impact on the management of patients in a clinical practice setting outside of clinical trials.
- AIM: Demonstration of the feasibility and reliability of deep molecular response assessment for all patients in Europe
- Survey aiming to provide insights on deep molecular responses after analysis of 5000 blood samples all over Europe driven by ELN (2013-2015)
- Samples will be accessed for Deep Molecular Responses through the established EUTOS MR4.5 Lab Network: all assessed laboratories that will receive EUTOS accreditation
- Primary objective: Demonstration of the access of all European patients to a standardized and validated detection of deep molecular response
Why Molecular Monitoring Survey (EUREKA) is clinically meaningful for the CML patients

Ethics committee approval
- 03.12.2013 Jena University Hospital (for German participants)
- Ethics approval for other countries pending
Inclusion criteria
- Male or female patients >18 years of age
- Patient with diagnosis of BCR-ABL positive CML in chronic phase (CP)
- Patient is receiving treatment with any TKI at registry entry, as per routine clinical practice
- Patient has been treated with one or more TKIs for a minimum of 24 months at registry entry
- Written informed consent
Sample and data collection
20 ml EDTA blood will be taken as part of the routine BCR-ABL monitoring of the patient. The blood sample (with full name), accompanied by the completed CRF form with some basic clinical parameters in anonymized format, should be sent by the attending physician to one of the local MR4.5-certificated labs (addresses see CRF and distributed kits). Blood can be sent once or repeatedly from the same individual with at least 10 weeks interval between samples over a period of 2 years. The laboratory performing the analysis of the sample will be responsible for inserting the CRF form together with the results of the analysis to an eCRF that will be then submitted to the EUREKA database (Fig. 1). The attending physician will receive a report of the PCR result from the MR4.5-certificated lab.

How to participate in the EUREKA registry
In order to include CML patients into the EUREKA registry please get in contact with EUREKA Trial Center (eureka@med.uni-jena.de) for detailed information.
Downloads
Germany
 Ethikvotum
(369KB)
Ethikvotum
(369KB)
International
 EUREKA registry protocol
(2.8 MB)
EUREKA registry protocol
(2.8 MB)
Contact Information
EUREKA Trial Center
eureka@med.uni-jena.de
Phone: +49 3641 9-396660
Fax: +49 3641 9-396669
Universitätsklinikum Jena
Klinik Innere Medizin II
Postfach
07740 Jena
Germany