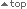Mean imatinib trough plasma levels in patients with or without CCyR or MMR 1
- European Treatment and Outcome Study
- Registry
- Molecular Monitoring
- Path to cure
- Spread of Excellence
Introducing Glivec blood-level testing
Table of contents
- Imatinib trough levels have been associated with clinical response
- Imatinib trough levels above 1000 ng/mL are associated with higher likelihood of cytogenetic and molecular responses
- Declining patient adherence can be an issue with long-term oral medications
- Imatinib blood-level testing can help you optimize patient outcomes
- Consider blood-level testing when:
- Imatinib blood level testing can help identify inadequate imatinib trough levels, and supports optimal management of patient treatment
- EUTOS for CML provides easy access to Imatinib blood-level testing
- Beyond Imatinib blood-level testing
- Indication and important safety information
- Important safety information
You can get the PDF-brochure, here
Imatinib trough levels have been associated with clinical response
Higher imatinib trough plasma levels have been associated with increased likelihood of achieving a complete cytogenetic response (CCyR) or major molecular response (MMR)1
- In patients treated with standard-dose GLIVEC® (imatinib), mean trough plasma levels were significantly higher in patients with CCyR or MMR than in those without CCyR or MMR, respectively 1

Imatinib trough levels above 1000 ng/mL are associated with higher likelihood of cytogenetic and molecular responses
- A plasma threshold of 1002 ng/mL was significantly associated with the presence of MMR (odds ratio 7.8; 95% CI: 2.64, 23.03; P<0.001) 1
- The majority (76%) of patients with MMR had trough plasma levels exceeding the 1002 ng/mL threshold; the majority (71%) of patients without MMR had trough plasma levels below the threshold 1
Imatinib trough plasma levels in patients with or without MMR 1

Declining patient adherence can be an issue with long-term oral medications
In a 14-month US study in the US, adherence began to decline after 4 months of treatment 2
- Persistency* declined from 94% at month 5 to 23% at month 14 (n=2921)
- Persistency averaged 256 days of therapy over 12 months
- Compliance rate was 75%†
Patients taking the recommended dose of imatinib (Glivec®) over 14 months 2

- Persistency declined from 94% at month 5 to 23% at month 14*
- Persistency averaged 256 days of therapy over 12 months 2
- Compliance rate was 75% 2†
*Persistency=time each patient stayed on therapy without significant gaps in refills.
†Compliance=medication possession ratio (apparent mg taken divided by mg prescribed).
Imatinib blood-level testing can help you optimize patient outcomes
Consider blood-level testing when:
- You have concerns about adherence
- The response to imatinib is less than expected
- Drug–drug interactions (DDIs) are suspected
- Side effects are unusually severe for the dose of imatinib taken
Imatinib blood level testing can help identify inadequate imatinib trough levels, and supports optimal management of patient treatment
- Results can offer a positive way to initiate an ‘evidence-based’ discussion of adherence with the patient
- Drug–drug interactions with concurrent medications may also cause inadequate imatinib levels
- Discuss other medications that the patient may be taking
- If adherence has declined due to side effects, note that most mild-to-moderate side effects can be managed with supportive care
EUTOS for CML provides easy access to Imatinib blood-level testing
((Note: Details below are an example only. CPO to adjust page according to local program specifics))
GLIVEC blood-level testing is available at no cost to you or your patients.
- Easy enrollment: Your local Novartis representative will be happy to provide you with the relevant forms to complete.
- Blood-level testing at no cost: EUTOS for CML will provide postage-paid, express-mail envelopes you can use to mail blood samples for testing. Imatinib blood-level testing will be performed at the central facility in Bordeaux.
- Obtaining results: You will receive test results directly from the center in Bordeaux
Sample Monitoring request form
you will find the monitoring request form in the download area
Beyond Imatinib blood-level testing
EUTOS for CML offers resources for your practice
A comprehensive package of supporting materials and activities is in development.
This resource is designed to make it as easy as possible for you to optimize imatinib therapy through blood-level testing.
It includes:
- A guide to participating in the EUTOS for CML blood-level testing program
- Medical education and slide kits
- Blood-level testing case studies
Indication and important safety information
Indication statement
Imatinib is indicated for the treatment of adult and pediatric patients with newly diagnosed Philadelphia-chromosome-(BCR-ABL) positive (Ph+) chronic myeloid leukemia (CML) for whom bone-marrow transplantation is not considered as the first line of treatment, and for adult and pediatric patients with Ph+ CML in chronic phase after failure of interferon-alpha therapy, or in accelerated phase or blast crisis.
Important safety information
Contraindications:
Hypersensitivity to imatinib or to any of the excipients
Precautions/Warnings:
Should be taken with food and a large glass of water to minimize the risk of gastrointestinal disturbances.
Beware of severe fluid retention. It is recommended that patients be weighed regularly. Regular monitoring of complete blood counts and liver function tests. Caution in patients with history of cardiac disease. Careful monitoring of patients with cardiac disease or risk factors for cardiac failure. Monitoring of TSH levels in thyroidectomy patients undergoing levothyroxine replacement. Should not be used during pregnancy unless clearly necessary. Should not be used by breast-feeding mothers
Interactions:
Caution with CYP3A4 inhibitors (e.g. ketoconazole, clarithromycin). Caution with CYP3A4 inducers (e.g. dexamethasone, rifampicin, phenytoin, carbamazepine, phenobarbital, St. John’s Wort).
Caution with substrates of CYP3A4 (e.g. triazolo-benzodiazepines, dihydropyridine calcium channel blockers, simvastatin, cyclosporin, pimozide), CYP2C9 (e.g. warfarin) or CYP2D6 (e.g. metoprolol). Caution with concomitant use of paracetamol/acetaminophen
Adverse reactions:
Very common: headache, nausea, vomiting, diarrhea, dyspepsia, abdominal pain, myalgia, arthralgia, muscle spasm or cramps, bone pain, dermatitis, eczema, rash, fatigue, weight increase
Created by: A. Hellenbrecht , generated 2008/01/03 , last changed: 2008/08/21